what are the important effects of sulfhydryl groups to protien structure and function
Abstract
Nutritional, structural, functional, and sensorial backdrop of protein isolate developed from salmon (Salmo salar), cod (Gadus morhua), and herring (Clupea harengus) by-products using the pH-shift method was studied. Office of the proteins in an emulsion organization in terms of viscoelastic properties was besides evaluated. Regardless of origin, the proteins showed satisfying nutritional value as reflected in their high essential amino acid content. The proteins contained significantly (p < 0.05) higher proportion of active sulfhydryl groups and surface hydrophobicity compared to whey and egg white protein reflecting conformational changes caused past the pH-shift process. Solubility, emulsion, and foaming capacity of the proteins showed a trend like to soy poly peptide and dependent on their origin. Cod protein had meliorate emulsion and foaming capacity than salmon and herring proteins which was in line with its loftier surface hydrophobicity and myosin heavy chain content. Emulsions adult from cod and salmon proteins showed essentially better viscoelastic backdrop, with college stability and viscosity compared to herring protein emulsions. Cod protein scored low for sensorial attributes related to lipid oxidation while herring protein showed loftier levels of fishy and rancid flavor and aroma. Altogether, results showed that the proteins from fish filleting past-products accept potential to exist used equally nutrient ingredients, but their application would be governed by their origin and sensorial properties.
Introduction
The fish processing industry produces huge amounts of side streams including heads, backbones, tails, viscera, blood, and trimmings which normally course more than than 50% of the fish weight (Shahidi 2006). Based on industrial activities, these by-products are currently mainly used as fauna feed by processing into fish meal or silage or in some cases even wasted (Aspevik et al. 2016). Some of these past-products similar the fish heads, backbones, tails, and trimmings tin can exist expert sources of high-value food-grade ingredients like fish protein. Nevertheless, the complex nature of these materials related to having high levels of heme-proteins, enzymes, and lipid too every bit difficulties in removing unwanted materials (e.g., bones, scales and connective tissues) have barricaded their successful market penetration (Abdollahi et al. 2016). These challenges have caused continuous research for finding novel alternative methods that can recover proteins from by-products, while retaining the protein functionality.
In this regard, acid and/or alkali metal solubilization followed by isoelectric precipitation, also called the pH-shift processing (Hultin et al., 2001), has been successfully recognized every bit a promising technique for direct protein recovery from unconventional complex aquatic raw materials, including gutted fish (Taskaya et al., 2009; Marmon and Undeland 2010) and seafood processing by-products (Chen and Jaczynski, 2007; Shaviklo et al. 2012). The procedure involves selectively extracting proteins from homogenized raw material using a high (> 10.5) or a low (< three.5) pH to solubilize the muscle proteins followed by centrifugation to separate the solubilized proteins from high and low density undissolved cloth. The solubilized proteins are so recovered using isoelectric atmospheric precipitation (normally pH 5.5) and dewatered by centrifugation or filtration. The recovered protein isolate can be mixed with cryoprotectants then frozen similar surimi or minced fish or might be directly dried into a fish protein pulverisation (FPP) for further utilization.
If the FPP be surimi of white muscle fish fillets, which have low fat and heme pigment content, an excellent source of high quality and concentrated fish poly peptide can be provided, which can be hands added to a wide range of food products every bit a good source of easily digestible amino acids (Santana et al. 2012). As a low fat, high protein content ingredient, it also has potential to be used as a binder, dispersing agent, and emulsifier in diverse re-structured nutrient products due to its strong interactions with other proteins and its good gelation capacity (Pires et al. 2009). Even so, in case of using fish processing by-products for production of FPP, little is known on how functional and sensorial properties are afflicted past the complexity of the by-products. Pires et al. (2012) reported acceptable functional properties for freeze-dried poly peptide isolate from Cape hake sawdust past element of group i-aid pH-shift process but Shaviklo et al. (2012) establish sensory attributes related to lipid oxidation in freeze-dried protein isolate of saithe cutting-offs generated with the same process. In both studies, nevertheless, non-bony by-products from white muscle fish resources were used. However, to the all-time of our knowledge, in that location is no study because the properties of FPPs produced from more complicated fish by-production like heads and backbones using the pH-shift method. Also, there is no a comprehensive study evaluating the issue of the fish species generating the by-products on the quality of the FPP developed using the pH-shift process; e.g., dark vs. white musculus fish and fatty fish vs. lean fish. Moreover, concluding awarding of the isolated proteins will exist adamant based on their functional properties like solubility, emulsion chapters, foaming chapters, and oil/h2o assimilation. These properties depend on the physicochemical and structural properties of the proteins and direct contribute to the taste, texture, and consumer acceptance of food products. Thus, understanding the effects of fish origin and the protein isolation process on nutritional and functional properties of fish protein isolate from the by-products is of cardinal importance, determining final application of the isolated protein.
Thus, the present report was aimed to evaluate nutritional, structural, functional, and sensorial properties of FPPs developed from poly peptide isolates generated with the pH-shift process from by-products of three dissimilar fish origins; salmon (Salmo salar), cod (Gadus morhua), and herring (Clupea harengus). In addition, properties of the developed FPPs were compared with dried soy, whey, and egg white albumin proteins to better understand potentials and limitations of the FPPs. An emulsion system was also developed from the iii FPPs and their viscoelastic properties were studied.
Materials and methods
Chemicals
Sodium hydroxide, hydrochloric acid, trichloroacetic acid (TCA), and sodium chloride were provided by Scharlau (Scharlau Co., Spain). Sodium dodecyl sulphate (SDS), β-mercaptoethanol (β-ME), glycerol, 5,v'-dithiobis(ii-nitrobenzoic acrid) (DTNB), eight-Anilino-1-naphthalenesulfonic acrid ammonium salt (ANS), and glutaraldehyde were purchased from Sigma-Aldrich Corp. (USA). Soy protein isolate (SPI), egg white protein (EWP, and whey protein isolate (WPI) were provided past Engelhardt co. (Gothenburg, Sweden).
Fish sample preparation
Fresh filleting by-product (head and tail on backbone) of cod (Gadus morhua) and salmon (Salmo salar) were provided by Fisk Idag Company (Gothenberg, Sweden). Herring (Clupea harengus) filleting by-product (head, backbone, and tail) were also provided past Swedish Pelagic Visitor. In the same day as processing, all fish by-products were fully covered with ice and were transported in the minimum of possible time. The samples were immediately minced using a table top meat mincer (C/E22 Due north, Minerva Omega group, Italy) equipped with a plate with three mm holes and pooled completely. Finally, the mince was frozen at − eighty °C in plastic zippo-lock bags for further employ.
Protein isolation and protein pulverisation development
Minced by-products were subjected to pH-shift processing post-obit the main principle described by Undeland et al. (2002) but with some modifications. Minced sample (800 chiliad) was homogenized with half dozen volumes of cold distilled h2o for two min at speed 6 using a Polytron Homogenizer (IKA, Brazil). The time of homogenization was primarily optimized, and the homogenizer was moved during mixing in a way that the whole solution was uniformly homogenized to assistance uniform pH adapted throughout the process. Then, the homogenate was adjusted to pH eleven.5 for cod and 12 for salmon and herring (based on a chief report on protein yield) using 2 M NaOH, respectively. The pH was automatically adapted with a titrator (907 Titrando, Metrohm AG, Zurich, Switzerland) in gear up pH mode with a maximum titration rate. The pH was monitored with a calibrated Hamilton double pore electrode (Bonaduz, Switzerland) coupled to the titrator. The pH-adapted slurry was allowed to stand in water ice for 10 min and then centrifuged at viii,500chiliad in a precooled (4 °C) Thermo Scientific Sorvall LYNX Superspeed Centrifuge (Thermo Fisher Scientific, Waltham, USA) for 20 min followed by recovery of the soluble protein. The soluble protein was then adjusted to pH v.5 as described above using the titrator with ten min holding time at pH 5.five on ice. A 2nd centrifugation pace at 8,500×1000 (4 °C, xx min) was then used to dewater the precipitated proteins. Recovered protein isolates were collected, and their pH was adjusted nether cold condition (< 4 °C) to vii.0 using cold ii N NaOH. The protein isolates were then lyophilized using a freeze-drier at − 56 °C for five days. Then, the samples were powdered using a coffee grinder and nigh 100 grand of each type of protein powder was obtained. The powders were stored at − 80 °C until further analysis.
Label of protein powders
Amino acid analysis
Amino acid composition of the protein powders was analyzed based on the method explained past Özcan and Şenyuva (2006) with some modifications. Six milligrams of freeze-dried powders was mixed with 4 ml of 6 N HCl and hydrolyzed at 110 °C for 24 h. Aliquots of the hydrolyzed samples were automatically injected to LC/MS (Agilent 1100 HPLC, Waldbron, Germany) with a Phenomenex column (C18 (ii) 250 μm × 4.vi μm × 3 μm), coupled to an Agilent 6120 quadrupole in the SIM positive way (Agilent Technologies, Germany) and compared confronting standard amino acids which were analyzed before. Sample training and injection were conducted in two replicates, respectively. With this method, tryptophan and cysteine were non recovered.
Active and total sulfhydryl groups measurement
Agile and full sulfhydryl groups content of the proteins were measured equally explained past Gong et al. (2015). Initially, 180 mg of protein was added to 30 mL Triseglycine buffer (0.086 Thousand Tris, 0.09 1000 glycine, 4 mM EDTA, pH viii.0) containing 8 M urea and stirred for 30 min at room temperature. Then, the solution was centrifuged at 10,000g for ten min and the supernatant was collected. To measure out the content of active sulfhydryl groups content, 4 mL of the diluted supernatant was added to 160 μL DTNB (4 mg/mL in the same buffer) and incubated for 15 min. The absorbance of the mixture was read at 412 nm using a spectrophotometer (Cary 60 UV–vis, Agilent technologies, Santa Clara, USA). The reagent (buffer + DTNB) was used as control, and the active sulfhydryl groups content was calculated using Eq. (1).
$$ \mathrm{Active}\ \mathrm{sulfhydryl}\ \mathrm{groups}\ \left(\upmu \mathrm{mol}/\mathrm{g}\right)=\frac{73.53\times \mathrm{Abs}\ \mathrm{at}\ 412\ \mathrm{nm}}{\mathrm{Sample}\ \mathrm{concentration}\ \left(\frac{\mathrm{mg}}{\mathrm{ml}}\right)} $$
(1)
where, Abs at 412 is the absorbance at 412 nm.
To measure total sulfhydryl group content, viii μL of β-ME was mixed with iv mL of the supernatant. After storage at 25 °C for 2 h, ten mL of 12% trichloroacetic acid (TCA) was added and the mixture was stored (1 h at 25 °C) again. The sample was then centrifuged at 10,000×thousand for 10 min and the pellet was rinsed with 5 ml of TCA (12%). The washing was repeated for three more times. Finally, the precipitate was dissolved in 6 mL of Tris-Glycine buffer, and iv ml of the diluted solution was mixed with 160 μL DTNB reagent (iv mg/mL), and its absorbance was determined at 412 nm. The above mixture without poly peptide was used as control. Full sulfhydryl group content was calculated using the same equation used for active sulfhydryl groups content.
Surface hydrophobicity assay
Surface hydrophobicity of the samples was measured co-ordinate the method explained by Timilsena et al. (2016). The protein solution (i mg/mL) was prepared in phosphate buffer (0.01 M, pH vii.0) and then centrifuged at x,000g for 20 min to remove any insoluble matter. The protein content of the fully dissolved fraction was determined, and protein dispersions with concentrations of 0.01–0.2% (w/v) were prepared by serially diluting with phosphate buffer (0.01 M, pH 7.0). So, four ml of each diluted sample was mixed with twenty μl of 8 mM ANS solution (solubilized in the aforementioned buffer). Afterwards 15 min incubation in darkness, the fluorescent intensity of the mixed solutions was determined using excitation and emission wavelengths of 374 and 485 nm, respectively. Fluorescent intensity of ANS lonely and diluted protein solutions without the probe were besides determined and were subtracted from the intensity of the sample. The calculated internet fluorescent intensity of the proteins was then plotted against their protein content. The initial slope of the plot was considered equally an index of average protein surface hydrophobicity.
Color measurement
Protein powders were subjected to colour measurement with a colorimeter (CR-400, Konica Minolta Sensing, Japan) as explained past Yin et al. (2011). The color was measured in the CIE L*a*b* color infinite past holding a probe directly against the lesser of a flat polystyrene plate containing protein samples. Five measurements of L*, a *, and b* were taken at different locations of the plates. Whiteness was also calculated according to formula (two):
$$ \mathrm{Whiteness}=100\hbox{-} \sqrt{{\left(100-L\right)}^2+{a}^2+{b}^two} $$
(2)
Bulk density measurement
Majority density of the protein powders was measured by filling a pre-weighed 10 mL graduated cylinder up to 10 mL marking with gentle tapping. The weight of the cylinder after filling was determined and used to calculate protein bulk density as g/mL.
Poly peptide water solubility
To evaluate the effect of pH on the solubility of the proteins in h2o, 1 grand of each protein pulverization was dissolved in 40 ml distilled water, and its pH was adapted to the range of ii–12 using 1 Northward HCl or NaOH solutions. Thereafter, the solutions were centrifuged at xv,000×g for 30 min at four °C. Protein content of the supernatant was afterward determined using a modified version of the Lowry method (Markwell et al. 1978), and relative solubility of the proteins was calculated based on the solubility at pH giving maximum solubility.
Emulsion activeness index and stability
Emulsion activity index (EAI) and emulsion stability of the protein powders were measured according the method explained by Ogunwolu et al. (2009). Initially, 300 mg of proteins was dispersed in thirty ml distilled h2o, and its pH was adjusted to 3, 5, 7, 9, and 11. Oil in h2o emulsion was then prepared by mixing the poly peptide solution with x ml of sunflower vegetable oil followed by homogenization at speed of 20,000×yard for 1 min. So, 50 μl of the emulsion in the lesser of the container was immediately and after 10 min pipetted into 5 ml of 1% SDS solution and vortexed for 20 s. Absorbance of the solution was adamant at 500 nm using spectrophotometer and the EAI and emulsion stability alphabetize (ESI) values were calculated using Eqs. (3) and (4), respectively.
$$ \mathrm{Emulsion}\ \mathrm{activity}\ \mathrm{index}\left({m}^two/g\right)=\frac{2\times 2.303\times {A}_0\times \mathrm{DF}}{C\times \varphi \times \theta \times 10000} $$
(iii)
where A 0 = measured absorbance at 500 nm; DF = dilution factor = 200; θ = path length of the cuvette = 1 cm; C = the initial concentration of protein (1000/mL); φ = the volume fraction of oil in the emulsion = 0.25.
$$ \mathrm{Emulsion}\ \mathrm{stability}\ \mathrm{alphabetize}\left(\min \right)=\frac{A_{ten}\times \varDelta t}{\varDelta A} $$
(4)
where A10 is the absorbance at ten min afterwards homogenisation; Δt = 10 min; and ΔA =A 0 −A 10.
Rheological analysis of emulsion containing fish proteins
To prepare an oil-in-water emulsion system, 3 grand of each fish protein powder was dispersed in 32 g distilled water with magnetic stirrer for 30 min. Then, 65 grand of sunflower oil was added to the dispersion, and emulsification was conducted with an IKA-polytron homogenizer at fourteen,000 rpm for 5 min every bit previously reported by Tomé et al. (2014). The emulsions were sealed and stored for 24 h at iv °C before color and rheological assay.
Dynamic viscoelastic properties of the emulsions were evaluated using parallel-plate geometry (25 mm plate diameter and 1 mm plate gap) mounted on a dynamic rheometer (Paar Physica Rheometer MCR 300, Anton Paar GmbH, Austria), operated in an oscillating and steady state mode at 20 °C using 0.5 g of emulsion samples. Frequency sweep test was conducted from 0.06283 to 628 rad/southward at a abiding shear stress inside the linear viscoelastic region of each emulsion. Oscillation sweep exam was done from 0.1 to thousand Pa at a constant frequency (vi.283 rad/due south) within the linear viscoelastic region of each emulsion. Steady-state menstruum test was conducted by measuring viscosity during logarithmic increasing shear stress from 0.1 to yard Pa and viscosity was plotted versus shear rate. Carreau model (5) was used for assay of the flow results:
$$ \eta =\frac{\eta_0}{{\left[1+{\left(\frac{\gamma }{\gamma^{\mathrm{c}}}\right)}^2\right]}^s} $$
(five)
where η 0 is the zero-shear rate limiting-viscosity, γ c is the critical shear charge per unit for the onset of the shear-thinning beliefs and s is a parameter related to the slope of the region.
Foaming chapters and stability
To measure foaming capacity and stability of the proteins, 250 mg of each protein pulverization was dispersed in 25 ml of distilled water (5-initial), and its pH was adjusted to iii, 5, 7, 9, and 11. The dispersion was and so homogenized using a Polytron homogenizer for 2 min at 10,000 rpm. The volume of the formed foam immediately later homogenization (5 1) and subsequently 60 min (V 60) was measured, and foaming capacity and foaming stability were calculated using Eqs. (6) and (7), respectively.
$$ \mathrm{Foaming}\ \mathrm{capacity}\left(\%\right)=\frac{V_1-{\mathrm{Five}}_{\mathrm{initial}}}{{\mathrm{5}}_{\mathrm{initial}}}\times 100 $$
(6)
$$ \mathrm{Foaming}\ \mathrm{stability}\left(\%\right)=\kern0.5em =\frac{V_{lx}-{V}_{\mathrm{initial}}}{V_{\mathrm{initial}}}\times 100 $$
(seven)
FTIR
Fourier transform infrared (FT-IR) spectroscopic analysis of the protein samples was carried out co-ordinate the method explained by Abdollahi et al. (2017). FT-IR spectra were obtained by placing fish protein and reference samples onto the crystal cell of a Nicolet 6700 spectrophotometer (Thermo Scientific, MA, Us) and scanning from four,000 to 400 cm−i at data acquisition charge per unit of 4 cm−ane per bespeak. All spectra were recorded at ambience temperature (25 °C) and 16 times scanning.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
The polypeptide pattern of the fish proteins, their initial raw textile, and the commercial reference proteins were investigated using SDS-PAGE according to the method of Laemmli (1970). Initially, 27 ml of 5% SDS solution was added to 3 g of each protein and homogenized using an Ultatorax at speed iii for ii min and heated at 85 °C for i h. Afterward cooling, the dissolved samples were centrifuged at five,000×m for xx min to remove undissolved residuals. The supernatant was mixed with an equal corporeality of Laemmli buffer (Bio-Rad, USA) containing v% β-mercaptoethanol and and then boiled for five min. Ten microliters of ladder (Prestained dual color standard, 10–250 kDa, Bio-Rad, USA) and 15 μg of protein from each sample were loaded onto a precast mini linear gels 4–twenty% (Bio-Rad, USA). Electrophoresis was conducted at a constant voltage of 205 V, using a Mini Poly peptide Ii unit (Bio-Rad, USA). After separation, 0.02% (due west/v) Coomassie Bright Blueish R-250 in fifty% (v/v) methanol and 7.5% (v/5) acerb acid was used for staining, and destaining was conducted with 50% methanol (five/v) and 7.5% (v/v) acerb acrid for 1 h. Finally, the gel was scanned in a GS-800 Calibrated Densitometer (Bio-Rad, Us).
Sensory analysis
Quantitative descriptive assay (QDA) method was used to evaluate sensorial attributes of the three FPPs and soy protein powders in the form of a ane% solution (1 g protein pulverization in 100 ml distilled water) every bit explained by Shaviklo et al. (2012). Protein solutions were prepared in Erlenmeyer flasks with spiral caps 1 h before analysis and stored cold. Vi skilled panelist from Engelhardt Visitor (Gothenburg, Sweden) were initially trained so asked to evaluate odour and season of the protein solutions in duplicate. Samples were coded with 3-digit random codes and were evaluated based on an unstructured scale (0–100%). Samples were randomly served to the panelist on a tray in individual booths, and they were asked to evaluate intensity of the attributes by smelling or tasting the solutions in terms of odor (dried fish, rancidity, TMA, fish oil) and flavor (dried fish, rancidity, fish oil, sweetness, bitterness, seaweed, TMA, and off-season). To clean and neutralize palate, water was provided for the panelists.
Statistical assay
All experiments in this inquiry study were carried out in Completely Randomized Design Test, and one-mode analysis of variance (ANOVA) and Duncan'south multiple range test were used to determine the significant differences between the variables. Differences with a probability value of < 0.05 were considered significant and all data were reported in the grade of mean ± SD. All experiments were run in triplicate (n = 3), except the mechanical properties, which was run in five replicates.
Results and discussions
Amino acid limerick
Every bit can exist seen in Table 1, FPPs of the 3 species had significantly (p < 0.05) higher total amounts of essential amino acrid (EAA) per g protein isolate compared to their initial raw materials. This effect can be related to the successful removal of collagenous impurities like the bone and skin, which contain loftier amount of non-essential amino acids including glycine and proline, during the pH-shift processing and concentration of the remained amino acids. However, salmon poly peptide powder independent lower amount of EAA compared with cod and herring protein powders. In addition, all FPP'due south had considerably lower corporeality of glycine and proline compared with their original raw materials. Higher corporeality of EAA was likewise reported in pH-shift produced protein isolates of rainbow trout (Y-C Chen et al. 2007) compared to their original raw material, i.eastward., gutted whole herring and trout by-products.
All EAA establish in the FPPs were well higher up the recommended requirement for adult based on WHO/FDA (WHO/FAO/UNU 2007). However, methionine, histidine, and phenylalanine did not run across the babe recommended intakes which are college than for adults. These results testify higher nutritional value of the developed FPPs in this study compared to previously reported data for protein pulverisation and isolates developed from hake cut-offs (Pires et al. 2012) and trout by-products (Y-C Chen et al. 2007) which only met the infant recommended intakes in their lysine and threonine content. Nutritional value in terms of total EAA was also higher in this study compared to that by (Sathivel et al. 2004) comprising protein powders developed by heat processing of arrowtooth flounder and herring.
The most abundant non-essential amino acid in the FPPs were glutamic acrid and aspartic acrid and when compared with the reference proteins, total amounts of EAA of the FPPs were higher than for SPI and well-nigh similar to EWP and WPI. The content of lysine in FPPs, which is a very important EAA, was 91–101 mg/k which was college than SPI (72 mg/1000) and EWP (81 mg/g) (which could help to make up for the lack of lysine in cereal foods (Zhong et al. 2016). )
Active and full sulfhydryl groups measurement
Sulfhydryl groups and disulfide bonds play important roles in some of the functional properties of proteins. As tin can be seen in Fig. 1a, the highest corporeality of total sulfhydryl groups was measured in WPI (114.3 μmol/m) and EWP (105.7 μmol/yard) while FPPs had significantly lower amount of total sulfhydryl groups (p < 0.05). Minimum total sulfhydryl group content was measured in herring protein pulverization (32 μmol/g). These results may reverberate the divergence in the amino acid limerick of the proteins from different origins having unlike amount of amino acids containing sulfur groups including methionine. On the other hand, FPPs showed considerably college ratio of agile sulfhydryl groups to total sulfhydryls. This ways that a big role of the sulfhydryl groups of fish proteins are exposed, while in SPI, WPI, and EGA most of the sulfhydryl groups were in the form of disulfide bonds. Sulfur-containing amino acids, specially methionine, are amongst of the most hydrophobic amino acids and are most ever institute in the interior of protein. This loftier ratio of active sulfhydryl groups may suggest that conformational changes in the FPPs occurred during the pH-shift solubilization, atmospheric precipitation, and/or freeze drying. It has been also previously reported that farthermost pHs used during pH-shift processing of fish proteins tin can crusade unfolding of proteins causing exposure of buried sulfhydryl groups (Abdollahi et al. 2017). Farther, information technology was reported that conformational changes and protein denaturation caused past high pressure and freeze-thaw cycles increased active sulfhydryl group content of walnut protein isolate (Qin et al. 2013) and SPI (Zhao et al. 2015), respectively.
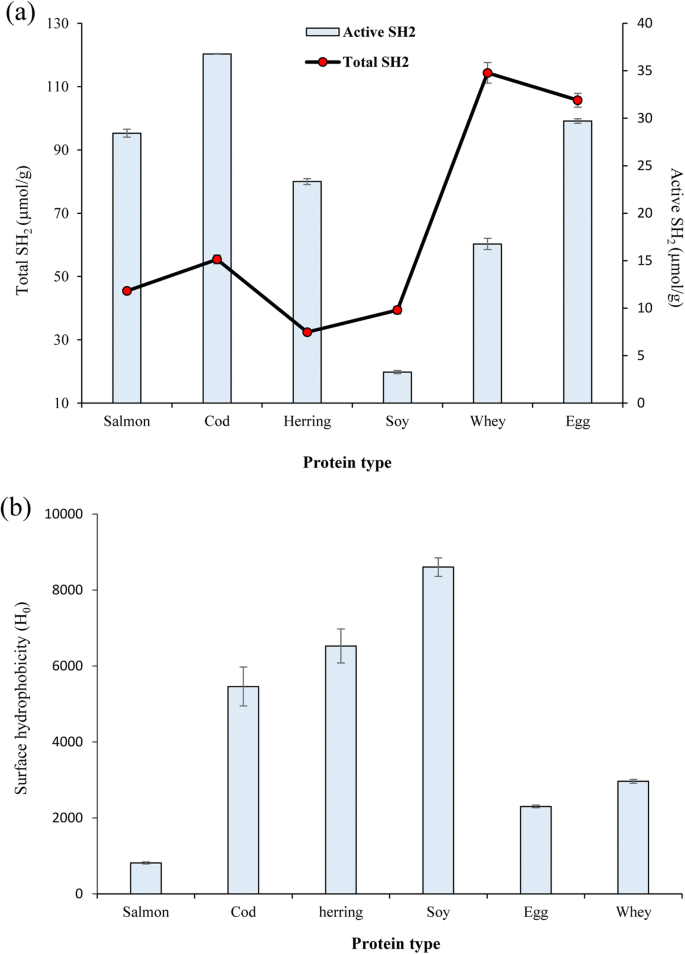
Active and total sulfhydryl groups (SH2) (a) and surface hydrophobicity (b) of fish proteins and the reference proteins
Surface hydrophobicity
Among the FPPs, the 1 from herring showed the highest surface hydrophobicity followed by cod protein powder, while surface hydrophobicity of salmon protein pulverization was significantly lower than that for herring and cod protein powders (p < 0.05) (Fig. 1b). The lower surface hydrophobicity observed in the cod protein powder compared to herring might exist related to the lower amount of conformational changes and thereby less exposure of internal hydrophobic side chains (Gong et al. 2015) caused past less extreme solubilization pH (pH xi.v) used for this species compared to herring (pH 12). Since both herring and salmon proteins were recovered using the same solubilization pH (12), the lower surface hydrophobicity observed in the salmon protein powder might be attributed to its higher refolding chapters after pH-readjustment. It might also be related to, e.g., hydrophobic assemblage of proteins to class more stable structure in salmon proteins after experiencing extreme pHs (Zhao et al. 2015). It has been also previously reported that too high protein denaturing factors like hydrostatic force per unit area in contrast with moderate pressures reduced surface hydrophobicity of proteins by causing hydrophobic interaction of the proteins (Qin et al. 2013). Surface hydrophobicity of all FPPs was lower than for SPI while herring and cod protein powders showed considerably college surface hydrophobicity than EWP and WPI. Similarly, Pires et al. (2012) reported lower surface hydrophobicity in hake protein powder compared to SPI. The lower surface hydrophobicity measured for EWP and WPI might also exist related to their different product and/or drying method.
Color and bulk density
Among the FPPs, the highest whiteness was measured in cod protein pulverization (71.83), which was significantly higher than the whiteness of salmon (66.09) and herring (54.75) poly peptide powders (p < 0.05) (Table 2). Cod protein powder showed a lite grey color while salmon and herring protein powders had brownish and nighttime brown appearance, respectively. The whiteness values of the cod and salmon poly peptide powders coincide with what was previously reported for powders made of Alaska pollock (64–76) (Sathivel and Bechtel 2006), merely were lower than Cape hake protein powder (78) (Pires et al. 2012). The lowest amount of whiteness was measured in herring protein powder which is most likely related to the high corporeality of heme-paint in the herring raw material and too the college amount remaining in the herring protein isolate (data not shown). These pigments tin can be oxidized during the precipitation at pH 5.5 to yield the dark-brown methemoglobin or metmyoglobin. In full general, the used reference protein powders had higher whiteness than FPPs with maximum of 87.17 for WPI. The major differentiating color parameter between the FPPs and the reference proteins was redness (i.e., a*). The FPPs showed redness values of three.five to vii.5 while it was < 0.8 in the three reference proteins. This is most likely related to the heme proteins of the fish FPPs, equally were discussed above for whiteness. The difficulty to completely remove heme pigments during the pH-shift procedure has been shown earlier using Hb fortified cod mince (Abdollahi et al. 2016). Yellowness index (i.e., b*) varied betwixt 14.41 for the herring poly peptide and 18.37 for the salmon protein.
Bulk density is a determinant factor in packaging requirements of protein powders. Bulk density of salmon protein pulverization (0.49 g/ml) was significantly lower than cod (0.lx g/ml) and herring (0.59 g/ml) poly peptide powders (p < 0.05) (Tabular array 2). It has been reported that bulk density depends on the combined furnishings of interrelated factors such as the intensity of attractive inter-particle forces, particle size, and number of contact points (Shao et al. 2014). The lower majority density of salmon protein pulverisation can be related to its lower protein content compared with cod and herring protein powders. The bulk density of the three FPPs was considerably lower than bulk density of FPP from saithe cut-offs (three.7–4.7 1000/ml), but were in the range of the bulk densities obtained for the reference proteins (0.48–0.sixty g/ml) used in this study.
Solubility of proteins in water
Solubility of protein powders in water at different pHs are summarized in Fig. 2a. FPPs and SPI showed a typical U-shaped solubility curve with minimum solubility measured at their isoelectric point. Solubility of FPPs at pH seven was 9–11%, which was higher than what was reported for hake protein pulverization (4%) (Pires et al. 2012) but lower than saithe protein isolate (Shaviklo et al., 2012) which can be related to different methods used for solubility measurement. By increasing solubilization pH to 10, the solubility of salmon and cod protein increased up to 62 and 53% which was considerably college than herring poly peptide solubility (29%). All FPPs showed solubility > 95% at pH 12 which was significantly (p < 0.05) college than SPI solubility (85%) at that pH. In full general, poly peptide solubility of the FPPs showed similar pattern every bit non-processed fish myofibrillar proteins, which have high solubility at very element of group i and acidic pHs and very depression solubility at ~ pH 5.5. Denaturation occurring during the pH-shift process and existence reflected in high surface hydrophobicity of the fish proteins might have afflicted solubility of the FPPs. Even so, compared to salmon (95%) and herring (81%), the cod poly peptide powder showed considerably lower solubility at pH 2 (50%). EWP and WPI showed very loftier solubility (> 80%) at all studied pHs. Solubility of proteins in h2o depends on several factor including surface characteristics of their amino acids, molecular weight, and conformational situation (Timilsena et al. 2016). For example, whey proteins are low molecular weight globular proteins remaining subsequently pH-aligning of milk to four.6 during the casein coagulation procedure. That fish proteins showed almost similar or better solubility pattern compared to SPI can open up upwardly for like application as SPI in food products.
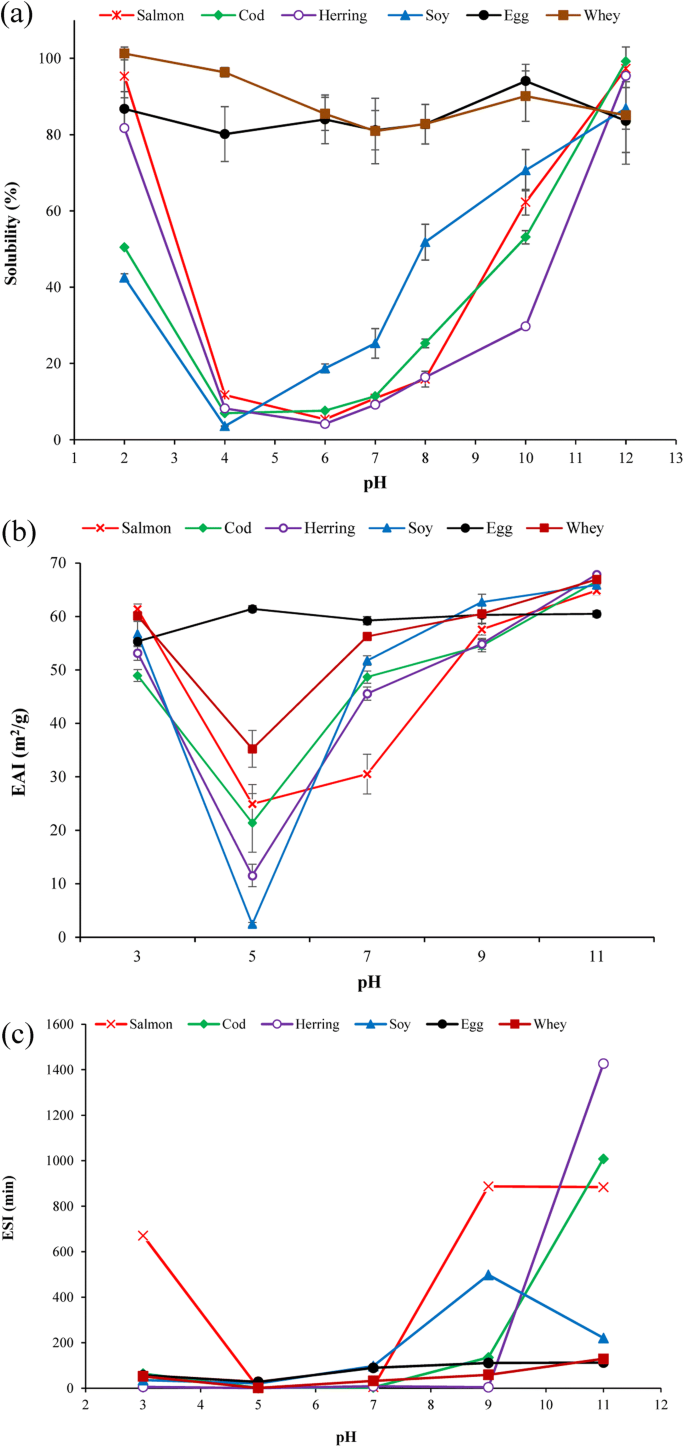
Water solubility (a), emulsion activeness index (EAI) (b), and emulsion stability index (ESI) (c) of fish proteins and the reference proteins as a function of pH.
Emulsion activeness and stability
Emulsification capacity tin can ascertain potentials of proteins for application in a wide range of emulsion-based food products. Emulsion activity alphabetize (EAI) and emulsion stability alphabetize (ESI) of FPPs and reference proteins as a function of different pHs are shown in Fig 2b, c. All poly peptide powders except EWP showed their minimum EAI at pH 5 which was around the isoelectric region and increased to a higher place and below this region. For fish and soy protein, this tin be related to their minimum solubility between pH 4 and half dozen which was shown in Fig. 2a. At pH 7 which was the initial pH of the FPPs, salmon protein powder (30 mtwo/k) showed considerably lower EAI than cod (48 grandtwo/g) and herring (45 one thousand2/g) samples which had EAI almost as good as SPI (51 m2/g). EAI of FPPs produced in this study was college than EAI reported for poly peptide pulverisation from Greatcoat hake sawdust (ten mii/g) produced using the pH-shift method (Pires et al. 2012). Emulsion capacity of protein will depend on their ability to adsorb on the oil-water interface. In one case captivated, the emulsifying amanuensis protects dispersed stage droplets from coalescence by forming a film at the oil-water interface and by reducing the interfacial tension (Phillips 1994). The initial absorption of proteins to the interface will be determined both by the proteins solubility that let protein reach to the interface and by its hydrophobicity to provide optimum contact of non-polar groups with the oil phase (Shevkani et al. 2015). Thus, the lower EAI of salmon protein powder tin can be related to its considerably lower surface hydrophobicity (Fig 1b). Similarly, Kristinsson and Hultin (2003) reported a very skilful relationship betwixt increase in surface hydrophobicity and emulsion activeness of pH-shift-treated cod myosin. When farther increasing pH to 9 and 11, the EAI of the FPPs increased (64–67 thousand2/g) and reached its maximum at pH 11 (> 64 thou2/k) which was even higher than EAI of EWP (60 gtwo/chiliad). This trend also coincides with the tendency that was seen in the FPPs solubility (Fig. 2a). Maximum EAI was measured for herring protein powders (67 one thousand2/g) which was followed by cod (66 grand2/one thousand) and salmon (64 k2/g). On the acidic side (pH two), notwithstanding, salmon protein pulverization showed maximum EAI (63 mtwo/g) which was followed by herring protein pulverization (53 m2/g) and the minimum was measured for cod protein powder (48%). This also correlated very well with the solubility of the FPPs and their hydrophobicity as shown previously. High emulsion capacity of FPPs might be related to the partial unfolding and denaturation of proteins experienced during the pH-shift process which provides rapid adsorption of the relatively hydrophobic globular head of the pH-treated protein to the nonpolar lipid globules (Panpipat and Chaijan 2017). We hypothesize that the process of conformational changes at the oil-h2o interface is due to loss in tertiary structure rather than secondary construction. This is because the interfacial energy at the oil-water interface is probably bereft to overcome the activation free energy bulwark for complete unfolding of poly peptide. The refolded proteins produced using the pH-shift procedure would therefore be at an advantage over the native proteins, every bit they already have partly unfolded third structure merely a native secondary construction. For myosin, a very abundant protein of all the FPPs, this would mean that the head is in a molten globular state (Kristinsson and Hultin 2003). However, the FPPs developed in this written report independent a mixture of myosin and other myofibrillar and sarcoplasmic proteins which can play role differently during emulsification process and should be considered.
ESI of the FPPs increased with increasing pH and was considerably higher than for all reference proteins at pH 11. Salmon poly peptide powder reached its maximum ESI at pH 9 while herring showed maximum ESI at pH 11, which was the only pH at which herring protein powder showed high ESI. In the acidic area, salmon poly peptide showed a big difference in ESI compared with the other FPPs. As mentioned, overall emulsion chapters of proteins depends to their ability to adsorb to the oil-h2o interface, but the stability of the formed emulsion (i.e., ESI) will be determined by the backdrop of the formed layer (Shevkani et al. 2015). In this manner, loftier percentage of active sulfhydryl groups measured in FPPs (Fig. 1a) might have facilitated formation of a stable interface pic layer and helped ESI compared to other proteins.
Dynamic rheology and colour of emulsions containing fish proteins
The results of frequency sweep tests of the emulsions, plotting storage modulus (M') and loss modulus (G") against frequency are shown in Fig. 3a. In all samples, G' and Chiliad" increased slightly with logaritmic increment of frequecy but G' was always higher than K" in all emulsions. Information technology means that the emulsions had a structured organisation like a viscoelastic solid. Pires et al. (2012) also reported similar results for reological properties of emulsion prepared from Cape hake protein pulverization but they found lower G' for the emulsion compared to the emulsions prepared from cod and salmon proteins in this study. The emulsion prepared from cod poly peptide pulverization showed the highest Chiliad' and G" value indicating that information technology had more structured arrangement compared with emulsions from salmon and herring protein powders (Tomé et al. 2014).
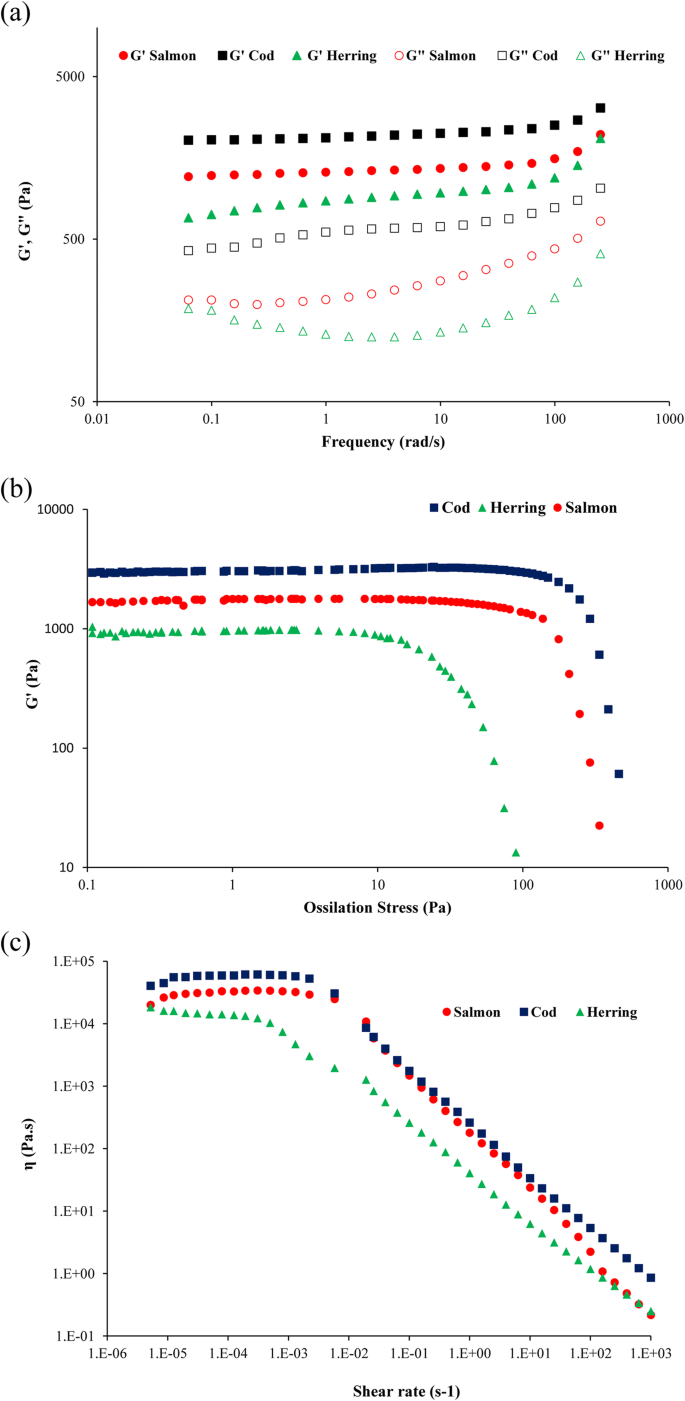
Frequency sweep (a), amplitude sweep (b), and menstruation curve (c) of emulsions made of salmon, cod, and herring proteins
The ossilatory stress sweep test can provide information almost the stability of the emulsion by increasing the amplitude of deformation or shear stress while the frequency is kept constant. As shown in Fig. 3b, the emulsion made with cod protein powder start to breakdown at 116 Pa which was follwed by those from salmon (81 Pa) and herring (ten Pa) proteins. That ways that the emulsions fabricated with cod and salmon protein powders showed considerably higher stabity compared with those made with herring protein powder. In addition, the cod protein powder containing emulsion showed the best viscoelastic properties and stability when loftier stress was applied on its structure.
In gild to evalute general flow behavior of the emulsions made of FPPs, their viscosity was mesured as a part of the shear rate and their and then called menses curve was shown in Fig. 3c. All emulsions showed a typical shear-thinning behavior with an initial Newtonian region with constant viscosity occurring at low shear rates followed by a region with straight-line decrease of viscosity with increasing shear charge per unit. Pires et al. (2012) and Tomé et al. (2014) reported similare menstruum curve with shear thinning behavior for emulsions made with Greatcoat hake poly peptide. The cod poly peptide powder containing emulsion showed the highest viscosity in the plateau region followed past the salmon protein powder containing emulsion, while the herring protein powder containg emulsion showed the everyman viscosity. The zero shear rate limiting viscosity (η 0) rsulted from Carreau equation (Fig. 3c) showed a dependency to the origin of the used poly peptide powder for emulsion preparation. Finally, cod protein powder resulted in the emulsion with the best viscoelastic properties, structure stability, and highest viscosity followed by salmon protein pulverisation, while herring protein pulverisation resulted the weakest emulsion. This can be related to the higher amount of myosin heavy chain with high molecular weight detected in cod poly peptide powders which play disquisitional part in emulsion chapters of myofibrillar proteins as discussed before (Fig. 5b). Equally stated before, it has been reported that college molecular weight proteins and high soluble protein content tin amend interactions between droplets in an emulsion (Yin et al. 2011). This is also in agreement with its college content of active sulfhydryl groups and high surface hydrophobicity.
Color attributes of the emulsions made of cod and salmon protein powders (Table two) showed high whiteness which shows high potential of these 2 powders for application in emulsion-type products. However, herring protein powder affected the whiteness negatively due to its night brown color.
Foaming properties
Foaming capacity (FC) and foam stability (FS) can clarify potential of proteins for awarding in certain nutrient systems, where aeration and overrun is needed, e.g., whipped toppings, broiled foods, and ice-cream mixes (Shevkani et al. 2015). FC and FS of FPPs and the reference proteins as a function of pH are summarized in Fig. 4a, b. FC of the FPPs showed a pH-dependent behavior with a minimum inside the isoelectric point region. FC of all FPPs increased by going far abroad from their isoelectric betoken region towards the acidic and alkali metal side, simply it was significantly college at alkaline pHs than acid ones (p < 0.05). At pH 7, cod protein showed the highest FC (65%) followed past salmon (53%) and herring (48%) protein powders which were significantly lower than FC of EWP and SPI (81%) (p < 0.05). Still, cod and herring protein powders showed FC (113 and 130%, respectively) higher than EWP (85%) and SPI (105%) at pH xi simply still lower than WPI (160%). Foam formation includes 2 main steps: (1) diffusion of solubilized proteins and adsorption to the gas-liquid interface to reduce surface tension and (2) poly peptide unfolding and orientation of hydrophobic regions to the gas phase and hydrophilic regions to the liquid phase to assume railroad train and loop formations (Lam et al. 2016). In this way, small-scale flexible proteins like whey and egg with high solubility tin reduce surface tension very quickly and brand foam. Thus, the college foaming capacity of FPPs at college pHs can be related to the increment in their solubility that permit them migrate quickly to the air/liquid interface and form the protective membrane (Shevkani et al. 2015). Also, the college FC of cod and herring poly peptide powder at pH 11 compared to SPI and EWP might be likewise related to their relatively higher solubility, protein content, and surface hydrophobicity at this pH every bit shown previously. It is hypothesized that protein conformational changes occurring during the pH-shift procedure, which increase their surface hydrophobicity, could also help them to unfold and orient their hydrophobic regions to the gas stage and hydrophilic regions to the liquid phase more quickly when they are solubilized enough. The lower FC of salmon protein isolate at pH 11, despite its high solubility at this pH, also coincide with its considerably lower surface hydrophobicity and its lower protein content compared to cod and herring proteins.
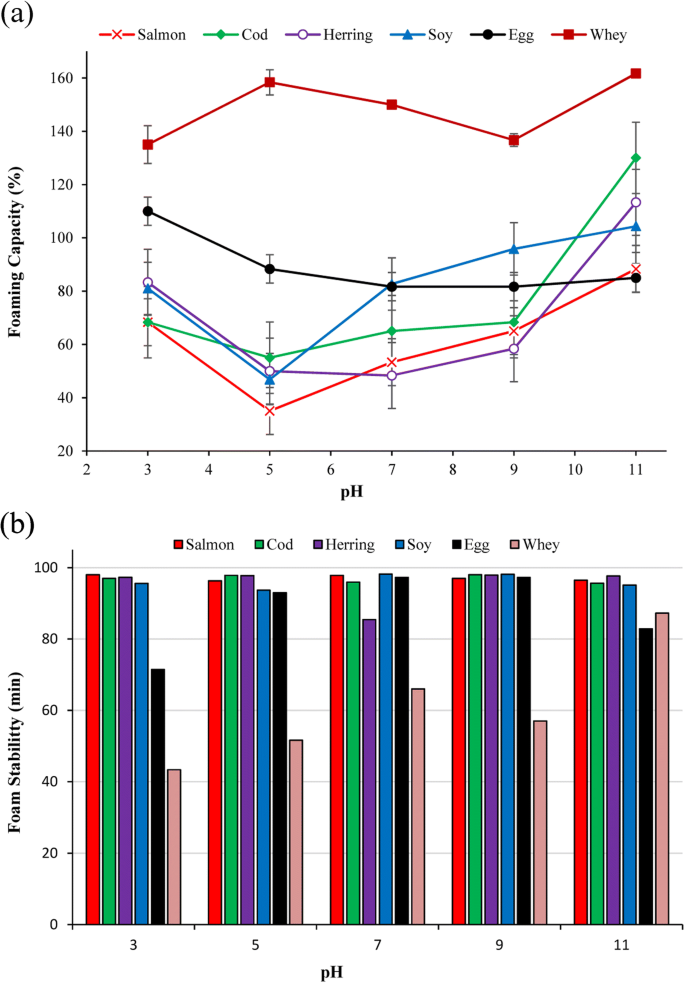
Foaming capacity (a) and foaming stability (b) of FPPs and the reference proteins as a part of pH
At all studied pHs, FPPs showed very loftier (> 95%) foam stability which was as expert as SPI just significantly higher than WPI and EWP (p< 0.05). In full general, stability of foams depends on the stability of the protein film formed in the gas-liquid interfacial layer and its gas permeability (Barać et al. 2011). Better foam stabilizers should exist able to course greater protein-protein interactions that volition increases viscosity and facilitate formation of a multilayer cohesive protein film at the interface (Panpipat and Chaijan 2017). It seems that the higher molecular weight fish poly peptide aggregates have been able to form a thicker, more cohesive and viscoelastic film around each gas bubble (Qin et al. 2013), thus showing very high foam stability compared with the reference proteins.
Molecular structure and polypeptide pattern of protein powders
FTIR spectra of protein powders from different origins are shown in Fig. 5a. All protein powders showed distinctive absorption bands at 3276-3282 cm−1 (Amide A, N-H, or O-H stretching), 1631-1637 cm-1 (Amide I, C = O, and C = Due north stretching), 1515-1517 cm−ane (Amide II, C-North stretching, and N-H bending), and 1232-12366 cm−1 (Amide Iii, PK6) (Ma et al. 2012; Chen et al. 2014). Amongst these peaks, the amide I absorption zone betwixt 1600 and 1700 cm−one can exist useful to evaluate the secondary construction of proteins as it is the sum of overlapping component bands: α-helix, β-sheet, and β-turn and random coils (Carbonaro and Nucara 2010). In herring protein pulverisation, the peak related to Amide I shifted to lower wavenumber (1633 cm−i) compared to the corresponding elevation from salmon and cod protein powders (1637 cm−one). Furthermore, in herring poly peptide powder, the peak related to Amide A shifted to lower wavenumber (3280 cm−1) compared with salmon and cod poly peptide powders (3282 cm−1). This might imply reduction in α-helix construction of myosin in herring protein pulverisation (Carbonaro and Nucara 2010). Raghavan and Kristinsson (2008) also reported that the pH-shift process affected the secondary structure of catfish myosin and caused an increment in its β-canvass structure. These two peaks were also establish in lower wavenumber in SPI, EWP, and WPI. These results may imply differences in the relative proportion of different secondary structure in the FPPs and the reference proteins. It has been shown that in full general, globular proteins have higher proportion of α-helix than β-sheet (Timilsena et al. 2016). In improver, in salmon and herring protein powders, a tiptop was seen at 1745 which was non detectable in other protein powders which could exist related to ester bonds between glycerol and fatty acids of lipids (Van der Weerd et al. 2005).
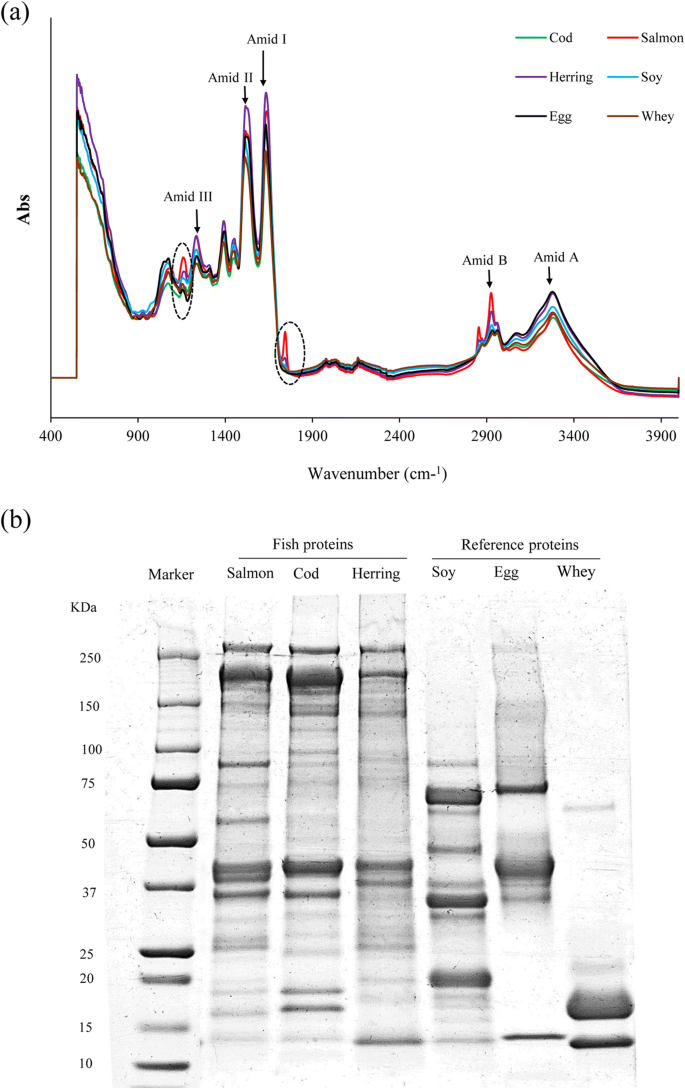
FTIR spectra (a) and polypeptide pattern (b) of FPPs and the reference proteins
Polypeptide pattern of FPPs and reference proteins are shown in Fig. 5b. With salmon and cod protein powders, myosin heavy chain (MHC) (~ 205 kDa) was the most abundant polypeptide followed by actin (~ 42 kDa). A dark shadow below the MHC in FPPs of all the three resources is too seen, which is not detectable in the polypeptide pattern of their raw materials. This might reflect slight proteolysis during the pH-shift procedure acquired by enzyme activity (Yongsawatdigul and Park 2004), an result not frequently reported during alkali-aided pH-shift processing of fish. Rather, proteolysis has been seen during acid-aided processing (Undeland et al. 2002). The maximum intensity of MHC was seen in cod protein isolate recovered at pH eleven.5. Since MHC plays a well-known instrumental part in the functional properties of muscle protein (Kristinsson and Hultin 2003), this may explain the college emulsion and foaming properties observed for the cod protein powders. Herring poly peptide isolates showed considerably lower MHC band intensity which may too explain its poor functional backdrop. This low MHC content might exist attributed to a high degree of hydrolysis occurring in the herring raw fabric already before the pH-shift process due to the well-known high proteolytic enzyme activeness in the nighttime muscle species.
As can be seen, WPI contained 2 major bands including β-lactoglobulin (~ 18 kDa) followed past α-lactoglobulin (14 kDa) and a slight band reflecting bovine serum albumin (~ 66 kDa). EWP showed a major band at around 45 kDa related to ovalbumin and another heavier band with less intensity at ~ 76 kDa related to ovotransferrin and a small band at around 14 kDa which could be related to lysozyme. SPI showed also a mixture of polypeptides with molecular weights less than 75 kDa including β-conglycinin fraction (α of 68 kDa, α′ of 72 kDa, and β of 52 kDa polypeptides) and the acid and basic polypeptide chains of glycinin (A of ~ 35 kDa, and B of ~ 20 kDa) (Nielsen 1985). The most noticeable difference between FPPs and reference proteins was the loftier number of band to a higher place 70 kDa which was institute in fish proteins just was not seen in soy, egg white, or whey protein. This divergence in molecular weight distribution may explain differences observed in emulsion and foaming capacity of the proteins. Usually, smaller proteins with lower molecular weight tin can migrate more quickly to the interfacial layer of oil/water or air/water to form a protective membrane. Notwithstanding, this property does not mean that such a poly peptide can form stable emulsion or cream.
Sensorial properties
As can be seen in Fig. vi, SPI had the lowest intensities of all odor and flavor attributes, except sweetness and bitterness values, which was higher in SPI than in salmon and cod poly peptide powders. FPPs by and large showed high intensities of fish oil and dried fish olfactory property and flavor but information technology was completely dependent on the origin of the FPP. Cod protein pulverization showed the lowest intensity of the all mentioned attributes followed past salmon protein powder. Notwithstanding, herring protein powder showed high intensity of lipid oxidation related scent and flavor and the highest intensities of stale fish and fish oil. The lipid oxidation aroma and season could result from oxidation taking identify in the herring samples during the bodily protein isolation (Undeland et al., 2005) because of the substantially higher amount of heme-protiens, well-known pro-oxidants which can even exist farther activated during the pH-shift process (Raghavan and Hultin 2009). Extra precautions are thus required to prevent lipid oxidation during pH-shift processing of by-products from dark muscle and fatty fish-like herring. However, Shaviklo et al. (2012) also constitute lipid oxidation related sensory attributes in freeze-stale protein isolate of saithe cut-offs, indicating that white fish by-products are not necessarily free of oxidation. Undeland et al. (2005) suggested a series of antioxidant mixtures suitable during acid aided pH-shift processing of herring fillets. These should exist evaluated also when using the element of group i process version, and also with other species. The medium to high levels of fish-related flavor and odor found in FPPs indeed ascertain the final awarding of the powders.
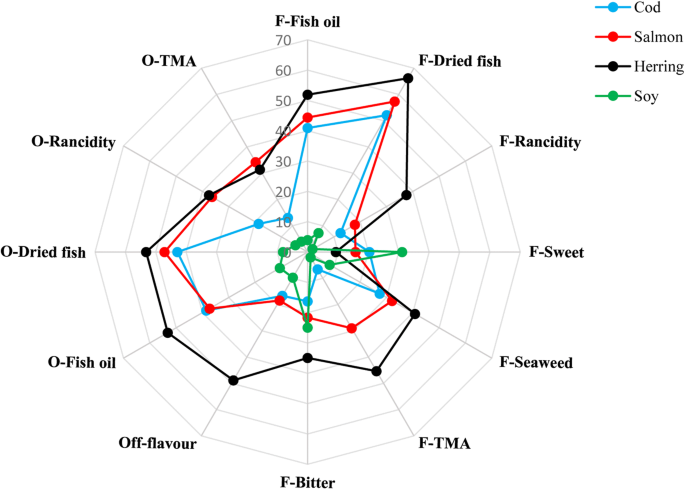
Sensorial attributes (F season, O odor) of fish proteins powders and soy poly peptide isolate in scale from 0 to 100 evaluated by skilled panelists.
Conclusions
Functional and sensorial properties of the FPPs were strongly dependent on the origin of the used raw materials. The FPPs independent 404–499 mg essential amino acids per gram of poly peptide, which was higher than their initial raw material and SPI. Cod and herring protein powders showed higher proportion of active sulfhydryl groups and surface hydrophobicity compared to WPI and EWP, which is affecting the functional properties of the FPPs. Emulsion and foaming capacities of the FPPs were related to their solubility and their origin and were equally adept as for EWP and SPI at high pHs. Cod protein powder showed meliorate emulsion and foaming capacity than salmon and cod protein powders which was in line with its high active sulfhydryl group content, surface hydrophobicity, and myosin heavy chain content. Emulsion organization adult from cod, and salmon poly peptide powders showed substantially better viscoelastic properties, with college stability and viscosity and whiteness, compared to herring protein powders. Sensorial properties of the FPPs were also dependent on their origin. Cod poly peptide powder obtained the all-time sensory score while herring protein powder showed high levels of fish and lipid oxidation-related flavor and odor. Overall, it seems that the recoevered FPPs from filleting by-products using the pH-shift process have potential to be used as food ingredients, but their application will be goverenced past their origin and sensorial properties.
References
-
Abdollahi, Grand., Marmon, S., Chaijan, Thousand., & Undeland, I. (2016). Tuning the pH-shift protein-isolation method for maximum hemoglobin-removal from blood rich fish muscle. Food Chemistry, 212, 213–224. https://doi.org/10.1016/j.foodchem.2016.05.165.
-
Abdollahi, 1000., Rezaei, M., Jafarpour, A., & Undeland, I. (2017). Dynamic rheological, microstructural and physicochemical properties of blend fish protein recovered from kilka (Clupeonella cultriventris) and silvery carp (Hypophthalmichthys molitrix) by the pH-shift process or washing-based technology. Food Chemistry, 229, 695–709. https://doi.org/10.1016/j.foodchem.2017.02.133.
-
Aspevik, T., Totland, C., Lea, P., & Oterhals, Å. (2016). Sensory and surface-active properties of protein hydrolysates based on Atlantic salmon (Salmo salar) by-products. Process Biochemistry, 51(eight), 1006–1014. https://doi.org/ten.1016/j.procbio.2016.04.015.
-
Barać, M., Cabrilo, Southward., Pešić, One thousand., Stanojević, Southward., Pavlićević, Grand., Maćej, O., & Ristić, N. (2011). Functional properties of pea (Pisum sativum, L.) protein isolates modified with chymosin. International periodical of molecular sciences, 12(12), 8372–8387. https://doi.org/10.3390/ijms12128372.
-
Carbonaro, M., & Nucara, A. (2010). Secondary structure of food proteins past Fourier transform spectroscopy in the mid-infrared region. Amino Acids, 38(3), 679–690. https://doi.org/10.1007/s00726-009-0274-3.
-
Chen, X., Chen, C. g., Zhou, Y. z., Li, P. j., Ma, F., Nishiumi, T., & Suzuki, A. (2014). Effects of high pressure processing on the thermal gelling properties of chicken breast myosin containing κ-carrageenan. Food Hydrocolloids, twoscore, 262–272. https://doi.org/10.1016/j.foodhyd.2014.03.018.
-
Chen, Y.-C., & Jaczynski, J. (2007). Protein recovery from rainbow trout (Oncorhynchus mykiss) processing byproducts via isoelectric solubilization/precipitation and its gelation properties as affected by functional additives. Journal of agricultural and food chemistry, 55(22), 9079–9088. https://doi.org/10.1021/jf071992w.
-
Chen, Y.-C., Tou, J. C., & Jaczynski, J. (2007). Amino acid, fatty acid, and mineral profiles of materials recovered from rainbow trout (Oncorhynchus mykiss) processing by-products using isoelectric solubilization/precipitation. Journal of nutrient science, 72(9), C527–C535. https://doi.org/10.1111/j.1750-3841.2007.00522.ten.
-
Gong, 1000. J., Shi, A. Chiliad., Liu, H. Z., Liu, 50., Hu, H., Adhikari, B., & Wang, Q. (2015). Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. Journal of Nutrient Engineering, 170, 33–40. https://doi.org/ten.1016/j.jfoodeng.2015.09.011.
-
Hultin, H. O., Kelleher, Southward. D., Feng, Y., Richards, M. P., Kristinsson, H., Undeland, I., & Ke, S. (2001). High efficiency protein extraction. Google Patents.
-
Kristinsson, H. G., & Hultin, H. O. (2003). Effect of low and high pH treatment on the functional properties of cod musculus proteins. Journal of Agricultural and Nutrient Chemical science, 51(17), 5103–5110.
-
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the caput of bacteriophage T4. Nature, 227, 680–685.
-
Lam, A. C. Y., Can Karaca, A., Tyler, R. T., & Nickerson, M. T. (2016). Pea protein isolates: structure, extraction, and functionality. Nutrient Reviews International, 00(00), 1–22. https://doi.org/10.1080/87559129.2016.1242135.
-
Ma, F., Chen, C., Sun, Chiliad., Wang, W., Fang, H., & Han, Z. (2012). Effects of high pressure and CaCl2 on properties of salt-soluble meat protein gels containing locust bean gum. Innovative Food Science and Emerging Technologies, fourteen, 31–37. https://doi.org/10.1016/j.ifset.2011.12.001.
-
Markwell, M. A., Haas, South. M., Bieber, L. L., & Tolbert, N. Eastward. (1978). A modification of the Lowry procedure to simplify protein conclusion in membrane and lipoprotein samples. Analytical biochemistry, 87, 206–210. https://doi.org/x.1016/0003-2697(78)90586-ix.
-
Marmon, S. G., & Undeland, I. (2010). Protein isolation from gutted herring (Clupea harengus) using pH-shift processes. Journal of agricultural and food chemical science, 58(19), 10480–10486. https://doi.org/x.1021/jf101057q.
-
Nielsen, N. C. (1985). Construction of soy proteins. New poly peptide foods (Us).
-
Ogunwolu, S. O., Henshaw, F. O., Mock, H. P., Santros, A., & Awonorin, Southward. O. (2009). Functional properties of poly peptide concentrates and isolates produced from cashew (Anacardium occidentale Fifty.) nut. Nutrient Chemistry, 115(3), 852–858. https://doi.org/10.1016/j.foodchem.2009.01.011.
-
Özcan, S., & Şenyuva, H. Z. (2006). Improved and simplified liquid chromatography/atmospheric pressure chemical ionization mass spectrometry method for the analysis of underivatized complimentary amino acids in various foods. Periodical of Chromatography A, 1135(2), 179–185. https://doi.org/ten.1016/j.chroma.2006.09.039.
-
Panpipat, Westward., & Chaijan, M. (2017). Functional properties of pH-shifted protein isolates from bigeye snapper ( Priacanthus tayenus ) head by-production. International Journal of Nutrient Properties, 20(three), 596–610. https://doi.org/10.1080/10942912.2016.1171778.
-
Phillips, L. G. (1994). Structure-function backdrop of food proteins. Bookish Printing.
-
Pires, C., Batista, I., Fradinho, P., & Costa, S. (2009). Utilization of element of group i-recovered proteins from cape hake by-products in the preparation of frankfurter-blazon fish sausages. Periodical of Aquatic Nutrient Product Technology, xviii(i–ii), 170–190. https://doi.org/ten.1080/10498850802629135.
-
Pires, C., Costa, Southward., Batista, A. P., Nunes, Chiliad. C., Raymundo, A., & Batista, I. (2012). Properties of protein powder prepared from Cape hake by-products. Periodical of Nutrient Engineering, 108(2), 268–275. https://doi.org/ten.1016/j.jfoodeng.2011.08.020.
-
Qin, Z., Guo, 10., Lin, Y., Chen, J., Liao, X., Hu, X., & Wu, J. (2013). Furnishings of high hydrostatic pressure on physicochemical and functional properties of walnut (juglans regia l.) protein isolate. Journal of the Science of Food and Agriculture, 93(5), 1105–1111. https://doi.org/10.1002/jsfa.5857.
-
Raghavan, South. & Kristinsson, H.Chiliad. (2008). Conformational and rheological changes in catfish myosin during alkali-induced unfolding and refolding. Nutrient Chemistry, 107, 385–398.
-
Raghavan, Southward., & Hultin, H. O. (2009). Outcome of diverse antioxidants on the oxidative stability of acid and alkali solubilized muscle protein isolates. Periodical of Food Biochemistry, 33(2), 163–175. https://doi.org/10.1111/j.1745-4514.2008.00202.10.
-
Santana, P., Huda, Northward., & Yang, T. A. (2012). Technology for production of surimi pulverization and potential of applications. International Food Research Journal, 19(4), 1313–1323. https://doi.org/10.1016/S0268-005X(03)00082-1.
-
Sathivel, S., & Bechtel, P. J. (2006). Backdrop of soluble poly peptide powders from Alaska pollock (Theragra chalcogramma). International Periodical of Food Scientific discipline and Technology, 41(5), 520–529. https://doi.org/10.1111/j.1365-2621.2005.01101.10.
-
Sathivel, Due south., Bechtel, P. J., Babbitt, J., Prinyawiwatkul, Due west., Negulescu, I. I., & Reppond, One thousand. D. (2004). Properties of poly peptide powders from arrowtooth flounder (Atheresthes stomias) and herring (Clupea harengus) byproducts. Journal of Agricultural and Food Chemical science, 52, 5040–5046. https://doi.org/10.1021/jf0351422.
-
Shahidi, F. (2006). Maximising the value of marine by-products. Woodhead Publishing.
-
Shao, D., Atungulu, K. Thousand., Pan, Z., Yue, T., Zhang, A., & Fan, Z. (2014). Characteristics of isolation and functionality of protein from tomato pomace produced with different industrial processing methods. Food and Bioprocess Technology, 7(2), 532–541. https://doi.org/x.1007/s11947-013-1057-0.
-
Shaviklo, One thousand. R., Thorkelsson, G., Arason, S., & Sveinsdottir, M. (2012). Characteristics of freeze-dried fish protein isolated from saithe (Pollachius virens). Journal of food scientific discipline and applied science, 49(3), 309–318. https://doi.org/x.1007/s13197-011-0285-4.
-
Shevkani, K., Singh, North., Kaur, A., & Rana, J. C. (2015). Structural and functional label of kidney bean and field pea protein isolates: a comparative study. Nutrient Hydrocolloids, 43, 679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024.
-
Taskaya, L., Chen, Y.-C., & Jaczynski, J. (2009). Functional properties of proteins recovered from silver carp (Hypophthalmichthys molitrix) past isoelectric solubilization/precipitation. LWT - Nutrient Science and Technology, 42(6), 1082–1089. https://doi.org/ten.1016/j.lwt.2009.02.007.
-
Timilsena, Y. P., Adhikari, R., Barrow, C. J., & Adhikari, B. (2016). Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Nutrient Chemistry, 212, 648–656. https://doi.org/10.1016/j.foodchem.2016.06.017.
-
Tomé, A. S., Pires, C., Batista, I., Sousa, I., & Raymundo, A. (2014). Poly peptide gels and emulsions from mixtures of Cape hake and pea proteins. Journal of the Scientific discipline of Nutrient and Agriculture, (April). doi: 10.1002/jsfa.6717
-
Undeland, I., Hall, Thou., Wendin, K., Gangby, I., & Rutgersson, A. (2005). Preventing lipid oxidation during recovery of functional proteins from herring (Clupea harengus) fillets by an acrid solubilization process. Journal of Agricultural and Food Chemistry, 53(fourteen), 5625–5634. https://doi.org/10.1021/jf0404445.
-
Undeland, I., Kelleher, South., & Hultin, H. (2002). Recovery of functional proteins from herring (Clupea harengus) light muscle by an acid or alkaline metal solubilization process. Periodical of agricultural and …, 7371–7379. http://pubs.acs.org/doi/abs/10.1021/jf020199u. Accessed 3 January 2015.
-
Van der Weerd, J., Van Loon, A., & Boon, J. (2005). FTIR studies of the furnishings of pigments on the crumbling of oil. Studies in Conservation, fifty(JANUARY), 3–22. https://doi.org/10.2307/25487713.
-
WHO/FAO/UNU. (2007). Protein and amino acid requirements in man nutrition. Globe Health System technical report series, (935), 1–265. doi:ISBN 92 4 120935 vi.
-
Yin, H., Wan, Y., Pu, J., Bechtel, P. J., & Sathivel, S. (2011). Functional backdrop of protein fractions of aqueduct catfish (Ictalurus punctatus) and their effects in an emulsion arrangement. Journal of Food Science, 76(three). doi: 10.1111/j.1750-3841.2011.02057.x
-
Yongsawatdigul, J., & Park, J. W. (2004). Effects of alkali and acid solubilization on gelation characteristics of rockfish muscle proteins. Journal of Nutrient Science, 69(7), 499–505. https://doi.org/ten.1111/j.1365-2621.2004.tb13642.ten.
-
Zhao, J., Dong, F., Li, Y., Kong, B., & Liu, Q. (2015). Issue of freeze-thaw cycles on the emulsion action and structural characteristics of soy protein isolate. Process Biochemistry, 50(10), 1607–1613. https://doi.org/10.1016/j.procbio.2015.06.021.
-
Zhong, S., Liu, S., Cao, J., Chen, Due south., Wang, W., & Qin, Ten. (2016). Fish protein isolates recovered from argent bother (Hypophthalmichthys molitrix) by-products using element of group i pH solubilization and precipitation. Journal of Aquatic Food Product Technology, 25(3), 400–413.
Acknowledgements
The authors are grateful to Engelhardt Company for kindly providing the reference proteins and collaboration in the sensory examination likewise as Fisk Idag AB and the Scandic Pelagic Ellös AB for kindly providing the fish processing by-products. The authors too would like to thank Prof. Johan Bergenholtz for his kind assist in rheological studies.
Funding
The research was financially supported past Vinnova (project no. 2014-03469).
Author information
Affiliations
Corresponding writer
Rights and permissions
Open Access This article is distributed under the terms of the Artistic Commons Attribution iv.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted employ, distribution, and reproduction in whatsoever medium, provided y'all give appropriate credit to the original author(s) and the source, provide a link to the Artistic Commons license, and indicate if changes were made.
Reprints and Permissions
About this article
Cite this commodity
Abdollahi, M., Undeland, I. Structural, functional, and sensorial properties of protein isolate produced from salmon, cod, and herring by-products. Food Bioprocess Technol eleven, 1733–1749 (2018). https://doi.org/10.1007/s11947-018-2138-x
-
Received:
-
Accepted:
-
Published:
-
Issue Appointment:
-
DOI : https://doi.org/10.1007/s11947-018-2138-x
Keywords
- Fish poly peptide
- Functional backdrop
- By-products
- Structural backdrop
- Protein isolate
Source: https://link.springer.com/article/10.1007/s11947-018-2138-x
0 Response to "what are the important effects of sulfhydryl groups to protien structure and function"
Post a Comment